What minerals are in limestone
In this essay, what minerals are in limestone, I will discuss the minerals that are present in limestone, the importance of those minerals, and the geological processes that result in the production of limestone.
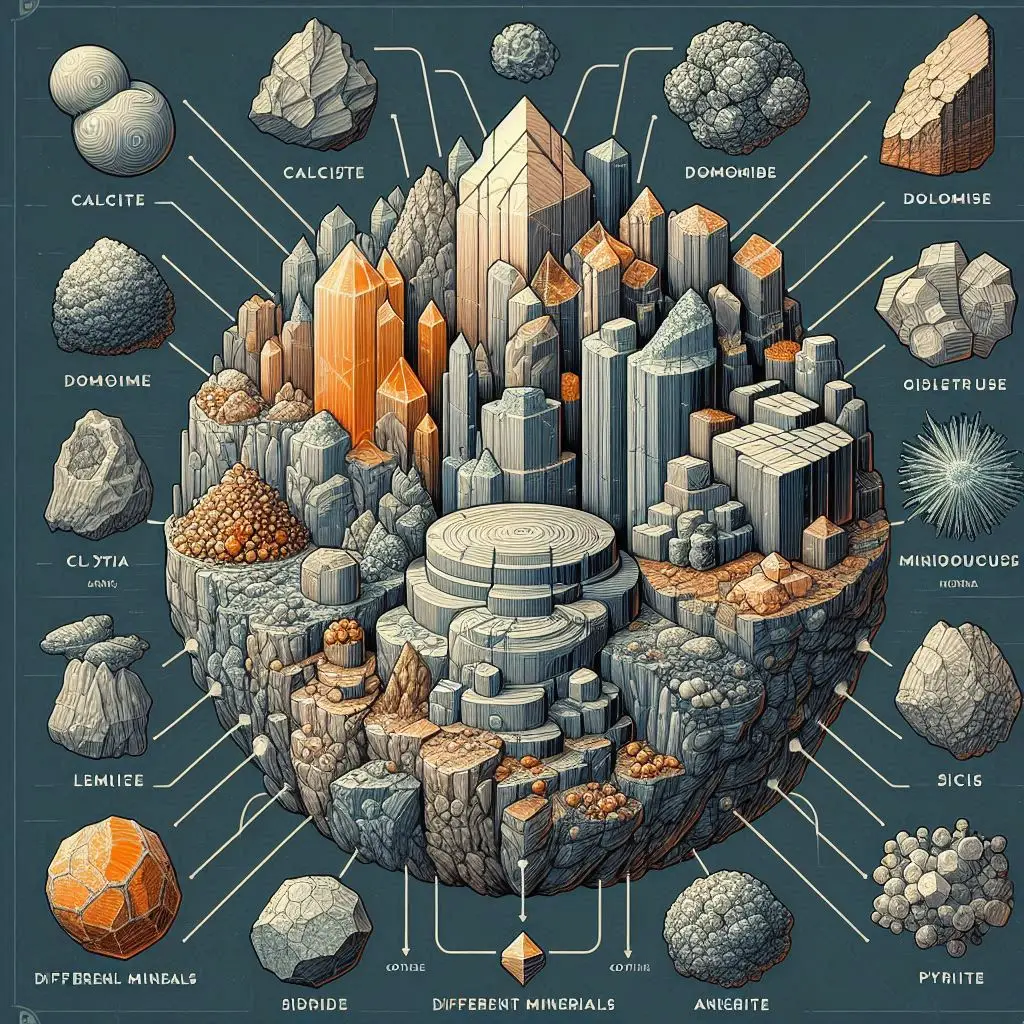
Minerals such as calcite, dolomite, clay minerals, silica, pyrite, siderite, ankerite, and different fossils are among the minerals that may be found in limestone. These minerals add to the rock's complex composition and the importance of the rock. The geological story that is retained in limestone formations is further enriched by sedimentary structures, secondary minerals, and geochemical changes. Not only does the study of limestone have practical applications, but it also provides important insights into the history of the Earth, paleontology, and the processes that occur in the environment. It is essential to strike a balance between the economic advantages of limestone and the protection of the environment to not only ensure the long-term viability of its use but also to protect the detailed geological records that are contained within this unique sedimentary rock.
Limestone is a kind of sedimentary rock that is mostly made up of the mineral calcite, which is a crystalline form of calcium carbonate (CaCO3). The fact that limestone may also include other minerals, contaminants, and fossils is one of the factors that contribute to the material's varied makeup.
Dolomite, clay minerals, silica, pyrite, and fossils are some of the minerals that are often found in limestone. Limestone is a sedimentary rock that is mostly formed of calcite, but it also frequently contains other minerals. The geological history of the Earth is reflected in its creation, which comes about as a result of the aggregation and lithification of the remnants of marine animals. In addition to being a major resource for a variety of businesses, limestone also contributes to our knowledge of the processes that occur on Earth and the ecosystems that existed in the past. The minerals that are included inside limestone have considerable commercial, agricultural, and scientific relevance. Not only can the study of limestone shed light on the history of the planet, but it also provides valuable insights into the usage of resources and sustainable methods for the future.
Formation of Limestone: The accumulation of marine creature remnants, including coral, tiny algae, and shells, results in the formation of limestone. Over time, these organic molecules are crushed and cemented together by minerals in a process called lithification. Calcite, which separates from the dissolved calcium carbonate in water, is the main mineral that forms limestone.
In limestone, minerals are :
Calcite (CaCO3): Usually making up more than 95% of the composition, calcite is the predominant mineral in limestone. It may appear in a variety of crystal shapes, including as prismatic, scalenohedral, and rhombohedral, depending on the circumstances under which it formed.
The mineral dolomite [(Ca, Mg)CO3] is also often present in limestone. This calcium-magnesium carbonate often develops as a secondary mineral during diagenesis, a process in which fluids rich in magnesium change preexisting calcite.
Clay Minerals: During sedimentary processes, clay minerals such as kaolinite and illite may be introduced into limestone. Certain limestones have a fine-grained texture because of these minerals.
Silica (SiO2): Chert nodules or other impurities may include silica in limestone. Radiolarians and diatoms are examples of silica-rich microfossils that may get lodged in limestone during its creation.
Pyrite (FeS2): Small, dispersed grains of iron sulfide minerals are often found in limestone. The presence of pyrite might be a sign of low oxygen levels during the deposition of limestone.
Fossils: Fossils are known to be preserved in limestone. Marine species' bones and shells can fossilize inside rocks, revealing important details about extinct life forms and their surroundings.
Relevance of Minerals Found in Limestone:
The Use of Calcite in Construction:
Because it contains a lot of calcite, limestone is a desirable resource for making crushed stone, dimension stone, and lime.
Dolomite in Agriculture: Acidic soils are neutralized and vital nutrients like calcium and magnesium are supplied for plant development via the use of dolomite-rich limestones.
Paleontologists use fossils to recreate historical ecosystems and comprehend evolutionary processes. The fossils found in limestone are essential archives of prehistoric life.
Limestone's participation in the carbon cycle is significant because it helps to control atmospheric CO2 levels by sequestering carbon dioxide via the precipitation of calcium carbonate.
Sedimentary Structures in Limestone: The many sedimentary structures seen in limestone provide light on the processes and conditions that led to the formation of the rock. Common characteristics of limestone include bedding, laminations, and cross-beddings, which show several phases of sedimentation as well as the impact of currents and waves. The physical and mechanical qualities of limestone formations are influenced by these structures, which also add to their perceived variety.
Siderite (FeCO3) and Ankerite (CaFe(CO3)2): These two iron-rich carbonate minerals may sometimes be found in limestone. These minerals are usually linked to certain geological circumstances, such as variable iron intake and shifting redox regimes. The limestone's color may be affected by siderite and ankerite, which can give it a yellow-to-brown tint.
Karst Topography and Secondary Minerals: Throughout geological time, limestone may dissolve in acidic water, forming unique landforms known as karst topography. Because of the reprecipitation of dissolved calcium carbonate, secondary minerals like as aragonite (an additional polymorph of CaCO3) and gypsum (CaSO4·2H2O) may be found in karst locations. The distinctive landscape of caves, subterranean rivers, and sinkholes seen in karst terrain is influenced by these secondary minerals.
Geochemical Variations: The source of the sediment, the chemistry of the water, and diagenetic processes may all affect the geochemical composition of limestone. The limestone structure may include phosphorus, manganese, and strontium, depending on the circumstances of the depositional environment. The appropriateness of limestone for various industrial purposes, including the manufacturing of cement, is influenced by geochemical differences in the material.
Human Use and Conservation: Because limestone is widely used in industry, agriculture, and building, more of it is being extracted, which raises questions about its effects on the environment and its preservation. Quarrying limestone may cause changes to the terrain, disturbance of habitats, and even problems with water quality. To strike a balance between the financial advantages of using limestone and environmental preservation, sustainable quarrying methods, land reclamation, and conservation initiatives are critical.

