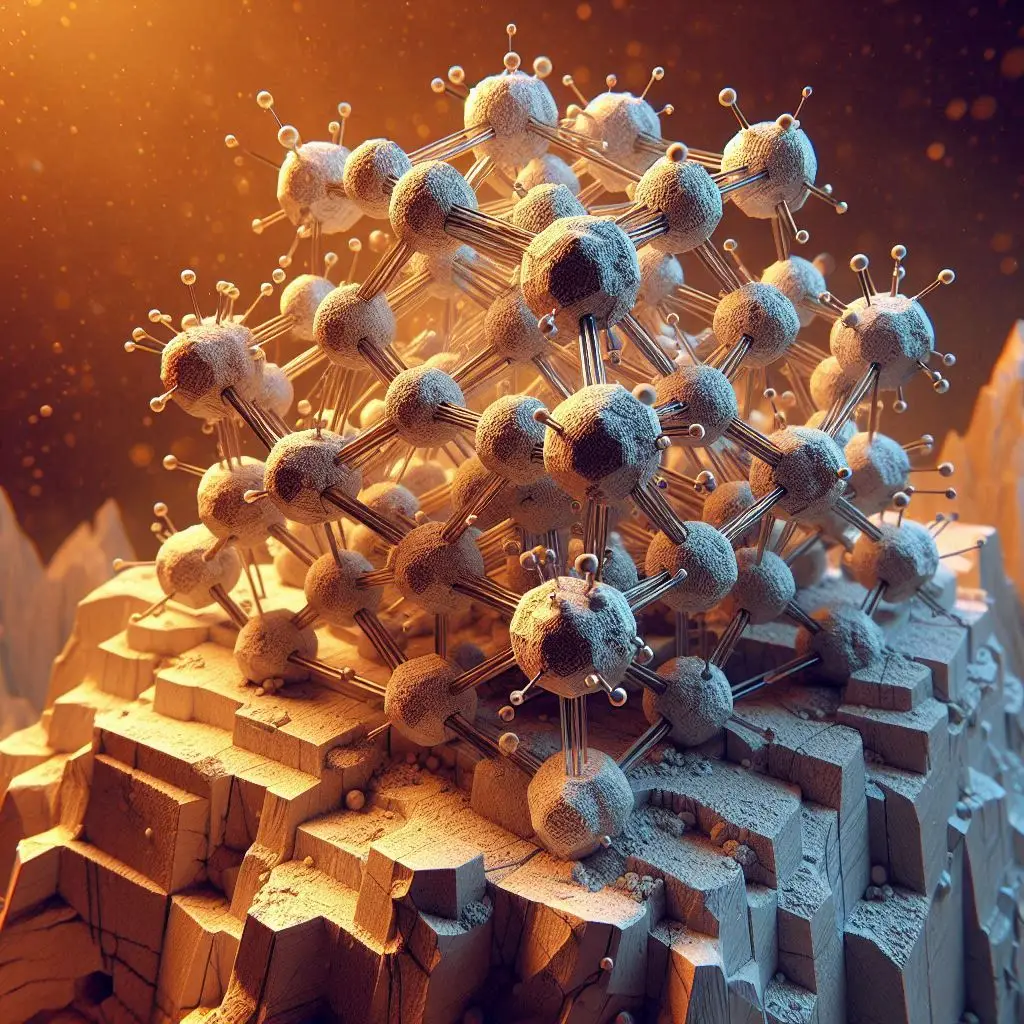
Kaolinite silicate structure
Let me talk about the kaolinite silicate structure.
The silicate structure of kaolinite is a fascinating topic that reveals the complex atom-to-atom dance inside its crystal lattice. This clay mineral is made up of a 1:1 ratio of tetrahedral and octahedral sheets, which provide it with special qualities that are used in a variety of industries. Kaolinite's structural complexity contributes to its adaptability and usefulness, as shown in its use in ceramics and as a catalyst support element.
Because of its easy cleavage into thin sheets due to the interaction of oxygen, silicon, and aluminum as well as the weak interlayer forces, kaolinite is a material that is in high demand across a variety of industries. Its attraction for water molecules and the hydrated kaolinite that results from this bond add still another level of intricacy to its behavior, affecting its interactions and stability in watery conditions.
As we continue to explore the field of materials science, the structure of kaolinite silicate serves as a reminder of the elegance and usefulness that may result from precisely arranging atoms. Future research on kaolinite and its derivatives might open up fresh possibilities for cutting-edge materials engineering applications and technological breakthroughs.
With broad ramifications for both scientific and economic fields, the kaolinite silicate structure is a complex wonder. Knowing the complicated crystal structure of kaolinite has opened up new uses, ranging from its use in conventional ceramics to cutting-edge nanotechnology. New aspects of the behavior of kaolinite and its derivatives are being discovered via continued research, which bodes well for future advances in materials science and technology.
Being a well-known member of the clay mineral family, kaolinite has a specific place in the world of silicate structures because of its unusual crystallographic characteristics and particular composition. With the chemical formula Al2Si2O5(OH)4, this aluminosilicate mineral exhibits a harmonic interaction between the atoms of silicon, aluminum, and oxygen, generating a complicated lattice that bestows exceptional qualities and uses.
Fundamentally, kaolinite is a phyllosilicate, with a layered structure that gives this mineral its intriguing characteristics. Tetrahedral and octahedral sheets, which are composed of silicon-oxygen tetrahedra and aluminum-hydroxide octahedra, respectively, are the fundamental building components of kaolinite. The 1:1 ratio in which these sheets organize themselves produces a clay mineral structure that sets kaolinite apart from other minerals in the clay mineral family.
The hexagonal lattice of linked silicon and oxygen atoms forms the tetrahedral sheets seen in kaolinite. With its four valence electrons, silicon forms a stable tetrahedral unit with three oxygen atoms sharing three electrons. Silicate minerals are distinguished by their tetrahedral sheet, which gives them toughness and structural stability.
The octahedral sheets, which are made up of aluminum atoms encircled by hydroxide ions, balance the tetrahedral sheets. These octahedral sheets combine with the tetrahedral sheets to produce the second layer of the kaolinite structure, like a sandwich. Aluminum's coordination with hydroxide groups adds to the structure's overall stability.
Weak van der Waals forces and hydrogen bonds hold the layers of kaolinite together, making it simple for the material to split into thin sheets. This characteristic is used in many industrial processes, such as the creation of paper, ceramics, and fillers for paints and plastics.
Kaolinite serves as an effective catalyst support in part because of its unusual crystal structure. In heterogeneous catalysis, kaolinite is useful because its exposed aluminum centers function as active sites for catalytic processes. To maximize kaolinite's catalytic characteristics in a variety of chemical processes, it is essential to comprehend the arrangement of its atoms.
Furthermore, kaolinite's behavior is greatly influenced by how it interacts with water molecules. Hydrated kaolinite is created when the hydroxyl groups on the surface of kaolinite easily establish hydrogen bonds with water molecules. This characteristic affects the stability, swelling behavior, and interactions of the mineral in aquatic settings with other compounds.
under addition to its structural details, kaolinite's qualities also affect how it behaves under heat, which affects how it is used in various thermal processes. When kaolinite is heated to high temperatures, changes to its crystal structure occur that affect its chemical and physical properties. When structural water is removed, which usually happens around 550°C, various changes start to happen. Metakaolin, a highly reactive substance with more surface area, is created when kaolinite is dehydroxylated. When making high-performance concrete, metakaolin is useful as an additional cementitious substance that improves strength and durability.
Due to its thermal properties, kaolinite is also an important component in the process of making alumina, a raw material used extensively in the aluminum industry. When kaolinite is calcined to temperatures higher than 900°C, two products are produced: mullite and alumina. The latter is an essential ingredient in the smelting of aluminum. Gaining an understanding of these thermal processes is essential to improving the efficiency of material manufacturing and optimizing industrial applications.
Furthermore, kaolinite's distinct electrostatic qualities add to its importance in a range of technical applications. In the crystal lattice, iron isomorphically replaces aluminum, causing a charge imbalance in the kaolinite structure. The mineral receives an electrical charge from this charge imbalance, which makes it beneficial for processes like wastewater treatment. The potential of kaolinite in environmental restoration is shown by the way its adsorption ability for different ions and compounds is used to remove pollutants from aqueous solutions.
Kaolinite's flexibility also extends to its use in geoscience, where its characteristics and existence provide important new understandings of geological processes. In tropical and subtropical regions, the weathering of rocks rich in feldspar is often linked to the creation of kaolinite. Understanding the distribution and features of kaolinite deposits may help interpret historical climatic conditions, patterns of erosion, and the region's geological past.
Within the field of nanotechnology, kaolinite's layered structure acts as a platform for the creation of nanocomposites. Materials with improved mechanical, thermal, and barrier characteristics may be created by intercalating different organic and inorganic compounds into the interlayer gaps inside the mineral. This creates opportunities for the development of sophisticated materials with specialized properties, such as reinforced polymers and innovative medication delivery systems.

