Methanol density g/ml cm3 kg
This article explains methanol density g/ml cm3 kg.
The density of methanol which is roughly 0.7918 g/mL, is a key characteristic that has wide-ranging effects on several sectors. It is a useful solvent because of its higher solvency and lower density than water. It is a viable alternative fuel with ramifications for transportation and is essential for both lab work and the production of many other goods. Furthermore, maintaining optimum coolant formulations in the automotive and industrial sectors depends on methanol's density. To maximize methanol's numerous uses and assuring the effectiveness and security of procedures across these sectors, it is crucial to comprehend and make use of methanol's density.
Methanol's density is a fundamental feature that determines its diverse usage in a variety of sectors. Because of its lower density than water, it is an ideal solvent for chemical operations. Furthermore, its potential as an alternative fuel is dependent on its density, which has an influence on the transportation and energy sectors. Precise density measurements are required in laboratories for correct testing, and methanol's density is critical in the automotive and industrial areas for maintaining optimum coolant compositions. Understanding and using methanol's density is critical for maximizing its many uses, which contribute to efficiency, safety, and innovation across a wide range of sectors.
The density of methanol is a fundamental characteristic that permeates numerous industries and applications. Its lower density compared to water makes it an indispensable chemical solvent. Its potential as an alternative fuel is contingent on its density, which influences the transportation and energy industries. In the laboratory, precision density measurements are required for accurate experimentation, and in the automotive and industrial sectors, the density of methanol is crucial for the maintenance of appropriate coolant formulations. Understanding and utilizing methanol's density is essential for optimizing its diverse applications, which contributes to efficiency, safety, and innovation in a wide range of industries.
The density of methanol is a fundamental characteristic that has significant implications across several industries and applications. The substance's reduced density in water makes it a vital solvent for several chemical processes. The viability of using it as a substitute fuel is contingent upon its density, which has significant implications for the transportation and energy industries. Accurate density measurements are crucial in laboratory settings to ensure exact experimentation. Similarly, in automotive and industrial environments, the density of methanol plays a critical role in maintaining appropriate coolant compositions. Comprehending and effectively using the density of methanol is crucial to maximizing its diverse range of uses, hence enhancing efficiency, safety, and innovation in several sectors.
Methanol density
In this discussion, I will delve into methanol's density and its significance.
Methanol, sometimes referred to as methyl alcohol or wood alcohol, is an achromatic and combustible liquid characterized by the molecular formula CH3OH. The component in question is a basic chemical substance that has many industrial uses. Consequently, comprehending and effectively using its density is of paramount importance.
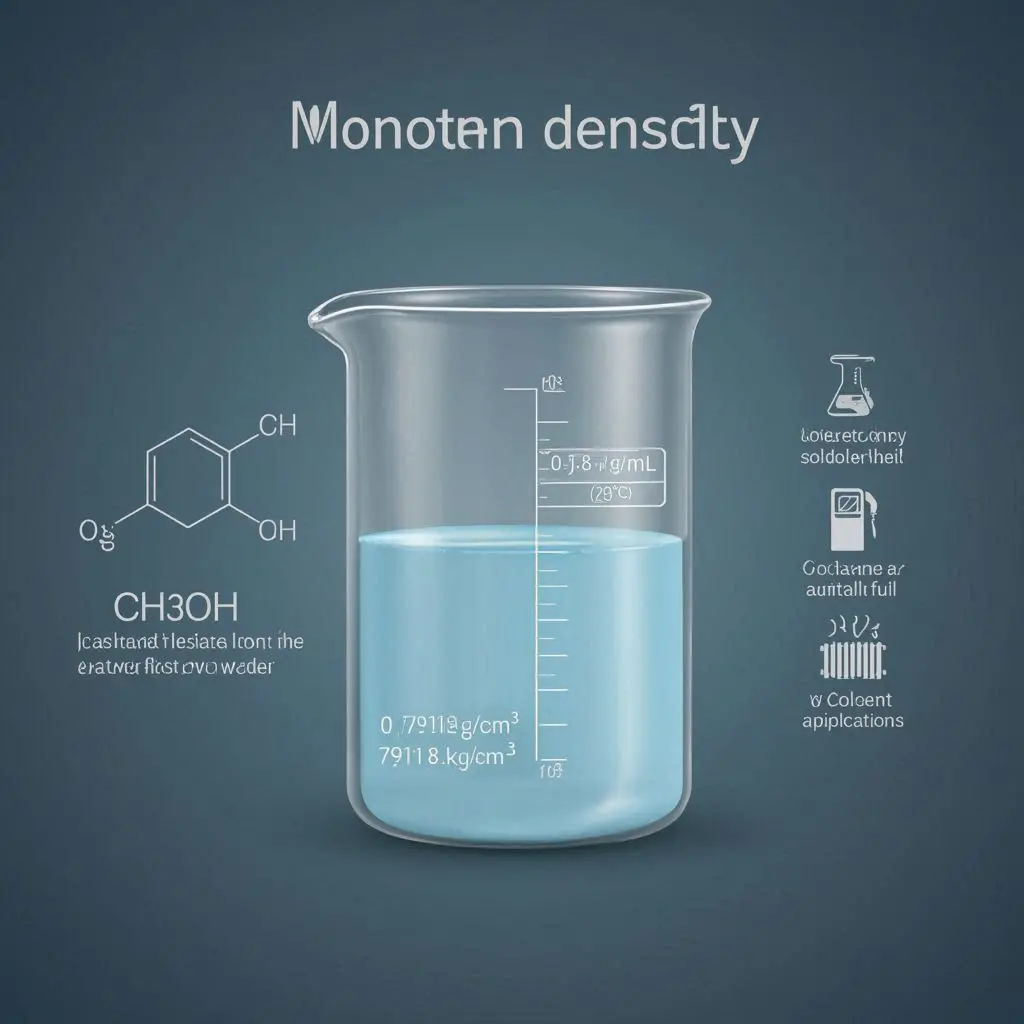
The concept of density refers to the mass of a material divided by its volume. Methanol has a density of roughly 0.7918 g/mL at a temperature of 20 degrees Celsius (68 degrees Fahrenheit). It may be inferred that the mass of one milliliter of methanol is about 0.7918 grams. The density of methanol is much lower compared to water, with water having a density of 1 g/mL at the same temperature. As a result, methanol exhibits a lower density compared to water, resulting in its ability to float over the water's surface upon their combination.
The density of methanol is of significant importance in several industrial sectors. One of the main uses of this substance is as a solvent in various chemical processes. Due to its low density, this substance is considered to be an optimal selection for the dissolution of a diverse array of organic and inorganic compounds. The efficient solubility of methanol plays a crucial role in chemical synthesis, pharmaceutical manufacture, and the creation of diverse consumer goods.
The automobile business also relies on methanol's density for many crucial purposes. Methanol serves as a viable substitute fuel in some vehicles and racing automobiles. The decreased density of methanol in comparison to gasoline or diesel results in a reduced mass per unit volume for a given quantity of methanol. The fuel economy of a vehicle may be influenced by this factor, since the use of lighter fuels necessitates less energy for propelling the vehicle, hence possibly leading to improved mileage.
In the field of laboratory research, the exact determination of methanol's density has significant importance in ensuring precise experimentation and analysis. This knowledge is crucial for scientists in the preparation of solutions and execution of chemical reactions using methanol. The determination of methanol density is crucial for scientists to accurately determine the required quantity of methanol for a certain experiment, hence guaranteeing precise outcomes.
Methanol is often found as a constituent in antifreeze solutions used in both automotive and industrial applications. The determination of methanol content in the coolant mixture is crucial in this particular application due to its density. Through an understanding of density, producers and mechanics possess the ability to devise the appropriate composition of coolant by proportionally blending methanol and water. This ensures the prevention of both freezing and overheating.
Methanol density g/ml
In this discussion, we'll explore methanol's density g/ml in-depth, its significance, and its impact on different sectors.
Methanol, also known scientifically as CH3OH, is a simple yet versatile chemical compound with numerous industrial applications. Its density, which is typically expressed in grams per milliliter (g/mL), is a fundamental property that forms the basis for many of these applications.
Understanding the density of methanol at standard conditions, which is approximately 0.7918 g/mL at 20 degrees Celsius (68 degrees Fahrenheit), is crucial. This indicates that one milliliter of methanol weighs approximately 0.7918 grams. Importantly, this density is lesser than that of water, which, at the same temperature, has a density of 1 g/mL. As a consequence, methanol is less dense than water, and this property has significant ramifications in numerous fields.
Methanol's use as a solvent in the chemical industry is one of its primary applications. Its relatively low density facilitates the dissolution of a vast array of organic and inorganic compounds. Methanol is favored for chemical synthesis, extraction processes, and the formulation of pharmaceuticals and consumer goods due to its ability to dissolve a wide variety of substances efficiently. Its density is crucial in determining the amount of methanol required for particular chemical reactions and processes.
Methanol has attracted attention as an alternative fuel source in the energy sector, particularly in the context of fuel cells and internal combustion engines. Its density is essential for this application. Although methanol contains less energy per unit volume than gasoline or diesel, its lower density can translate into weight and efficiency advantages for vehicles. Less energy is required to transport lighter substances, which could enhance fuel economy.
The laboratory sciences rely largely on precise measurements, and the density of methanol is no exception. The preparation of solutions, the determination of concentrations, and the execution of chemical experiments all require precise density measurements. Researchers and scientists use the known density of methanol to calculate the exact amounts required for specific experiments, thereby ensuring the accuracy and reliability of their findings.
In addition, methanol is commonly used as an antifreeze component in automobiles and industrial processes. The density of a substance is a crucial factor in formulating the proper refrigerant mixtures. By understanding the density of methanol, manufacturers and mechanics can ensure that the concentration of methanol in the coolant mixture falls within the desired range, thereby preventing freezing and overheating problems.
Methanol density in g/cm3
In this discussion, we will explore methanol's density in g/cm 3, its significance, and the wide-ranging implications of this crucial property.
Methanol, chemically represented as CH3OH, is a versatile and important substance in many industries, and one of its most important qualities is its density, which is commonly stated in grams per cubic centimeter (g/cm3).
Methanol has a density of roughly 0.7918 g/cm3 under typical circumstances (20 degrees Celsius or 68 degrees Fahrenheit). This number represents the weight of one cubic centimeter of methanol, which is roughly 0.7918 grams. Methanol has a lower density than water, which has a density of 1 g/cm3 at the same temperature. This trait has far-reaching repercussions in a variety of sectors and applications.
Methanol is widely used as a solvent in the chemical industry. Because of its low density, it is an excellent option for dissolving a broad variety of organic and inorganic compounds. The capacity to dissolve diverse compounds effectively is critical in chemical synthesis, pharmaceutical production, and the manufacture of several consumer items. The density of methanol is critical in estimating the amount of methanol necessary for various chemical processes and reactions.
Methanol has emerged as a possible alternative fuel in the energy industry, notably in fuel cells and internal combustion engines. The density of methanol is important in this situation. While methanol contains less energy per unit volume than gasoline or diesel, its reduced density may contribute to weight and efficiency benefits. Lighter fuels need less energy for transportation, which might result in increased fuel efficiency and lower emissions.
Precision measurements are critical in laboratory research, therefore the density of methanol is critical. Density measurements must be precise for making solutions, determining concentrations, and conducting chemical experiments. The known density of methanol is used by researchers and scientists to compute the precise quantities required for certain studies, assuring the correctness and trustworthiness of their findings.
Methanol is also extensively utilized as an antifreeze component in automotive and industrial applications. Its density is crucial in selecting the proper coolant mixes. Understanding the density of methanol enables manufacturers and mechanics to create coolant solutions with the proper amounts of methanol and water, successfully avoiding freezing and overheating.
Methanol density kg/m3
In this exploration, I will delve into the density of methanol in kg/m3, its significance, and the extensive implications of this vital property.
A crucial component of methanol, a simple but adaptable chemical substance symbolized by CH3OH, is its density, which is commonly given in kilograms per cubic meter (kg/m3). This density is one of methanol's essential features.
At typical circumstances, at 20 degrees Celsius (68 degrees Fahrenheit), methanol has a density of around 791.8 kg/m3. As a result, methanol has a mass of about 791.8 kilograms per cubic meter. It's significant that water, which has a density of 1000 kg/m3 at the same temperature, has a lower density than methanol. This specific trait has broad ramifications for many other fields and applications.
Methanol is often used as a solvent in the chemical industry, which is one of its main uses. It is a great option for dissolving a broad range of organic and inorganic compounds due to its comparatively low density. In the production of pharmaceuticals, consumer goods, and chemical synthesis, the effectiveness with which it dissolves different compounds is crucial. The exact amount of methanol needed for a given chemical reaction or process depends on the methanol's density.
The density of methanol is important in the energy sector as well. It has drawn interest as a possible alternative fuel, especially for internal combustion engines and fuel cells. In this situation, methanol's density is important. Methanol has a lower energy density than conventional fuels like gasoline or diesel, although this might have benefits like reducing vehicle weight and perhaps enhancing fuel economy. Lighter fuels use less energy during transportation, improving mileage and lowering pollutants.
Precision is crucial in laboratory research, and the density of methanol is no different. For making solutions, figuring out concentrations, and carrying out chemical experiments, accurate measurements of density are essential. The reliability and precision of their findings are increased by using the known density of methanol to compute the precise quantities required for each tests.
Additionally, methanol is a typical ingredient in antifreeze solutions used in both industrial and automotive applications. A crucial factor in creating the ideal coolant mixes is the density of the methanol. Manufacturers and mechanics may successfully avoid freezing and overheating problems by ensuring that the concentration of methanol in the coolant mixture is within the acceptable range by knowing the density of methanol.

